Textbook Question
Determine the percent ionization of a 0.125 M HCN solution.
1631
views
1
comments
 Verified step by step guidance
Verified step by step guidance
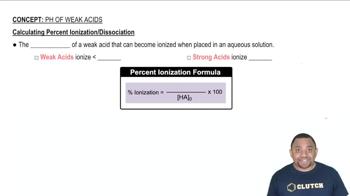
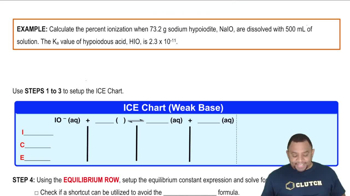

Determine the percent ionization of a 0.125 M HCN solution.
Determine the percent ionization of a 0.225 M solution of benzoic acid.
Calculate the percent ionization of a formic acid solution having the given concentration. b. 0.500 M
Calculate the percent ionization of a formic acid solution having the given concentration. c. 0.100 M
Calculate the percent ionization of a formic acid solution having the given concentration. d. 0.0500 M