Here are the essential concepts you must grasp in order to answer the question correctly.
Ionization of Weak Acids
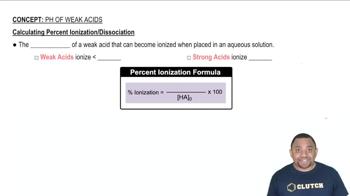
Weak acids, like hydrocyanic acid (HCN), do not completely dissociate in solution. Instead, they establish an equilibrium between the undissociated acid and its ions. The degree to which a weak acid ionizes is crucial for calculating properties such as pH and percent ionization.
Recommended video:
Calculating Percent Ionization of Weak Acids
Equilibrium Constant (Ka)
The acid dissociation constant (Ka) quantifies the strength of a weak acid by measuring the extent of ionization at equilibrium. For HCN, the expression for Ka is derived from the concentrations of the products (H+ and CN-) and the reactant (HCN) at equilibrium, which is essential for calculating percent ionization.
Recommended video:
Percent Ionization Calculation
Percent ionization is calculated by taking the concentration of ionized acid at equilibrium, dividing it by the initial concentration of the acid, and multiplying by 100. This metric provides insight into the strength of the acid and its behavior in solution, which is vital for understanding the properties of HCN in this context.
Recommended video:
Calculating Percent Ionization of Weak Acids