Here are the essential concepts you must grasp in order to answer the question correctly.
pH and pOH
pH is a measure of the hydrogen ion concentration in a solution, defined as the negative logarithm of the hydrogen ion concentration (pH = -log[H+]). A lower pH indicates a higher concentration of hydrogen ions, which is typical for acidic solutions. Understanding pH is crucial for calculating the concentration of ions in weak acid solutions.
Recommended video:
Weak Acids and Ionization
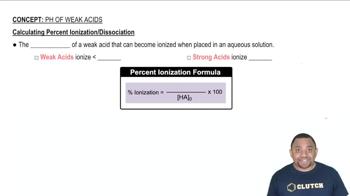
Weak acids do not completely dissociate in solution, establishing an equilibrium between the undissociated acid (HA) and its ions (H+ and A-). The degree of ionization is essential for calculating the acid ionization constant (Ka), which quantifies the strength of the acid. The equilibrium expression for a weak acid is given by Ka = [H+][A-]/[HA].
Recommended video:
Calculating Percent Ionization of Weak Acids
Acid Ionization Constant (Ka)
The acid ionization constant (Ka) is a quantitative measure of the strength of an acid in solution. It reflects the extent to which an acid donates protons to water, with larger Ka values indicating stronger acids. Calculating Ka involves using the concentrations of the ions at equilibrium, which can be derived from the initial concentration and the pH of the solution.
Recommended video:
Characteristics of Ka and Kb