Here are the essential concepts you must grasp in order to answer the question correctly.
Capillarity
Capillarity, or capillary action, is the ability of a liquid to flow in narrow spaces without the assistance of external forces. This phenomenon occurs due to the adhesive forces between the liquid and the solid surface of the tube, as well as the cohesive forces within the liquid itself. In this case, water exhibits strong adhesive forces with glass, leading to a higher rise compared to hexane.
Recommended video:
Intermolecular vs Intramolecular Example
Surface Tension
Surface tension is the property of a liquid that causes it to behave as if its surface is covered with a stretched elastic membrane. It arises from the cohesive forces between liquid molecules. Water has a high surface tension due to strong hydrogen bonding, which contributes to its ability to rise higher in the glass tube compared to hexane, which has weaker intermolecular forces.
Recommended video:
Intermolecular Forces and Properties
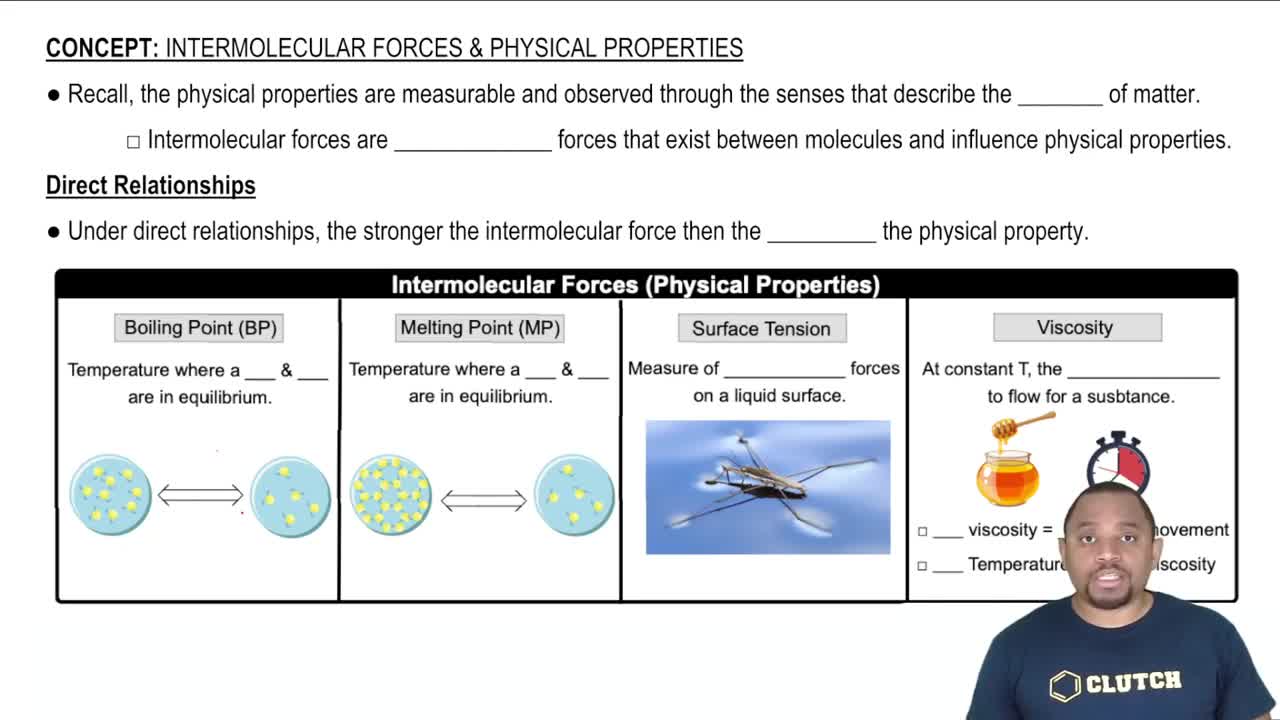
Intermolecular Forces
Intermolecular forces are the forces of attraction or repulsion between neighboring particles (atoms, molecules, or ions). These forces significantly influence the physical properties of substances, including their boiling points, melting points, and capillary action. Water, with its strong hydrogen bonds, has greater intermolecular forces than hexane, resulting in a more pronounced capillary rise in the glass tube.
Recommended video:
Intermolecular vs Intramolecular Forces
 Verified step by step guidance
Verified step by step guidance