Here are the essential concepts you must grasp in order to answer the question correctly.
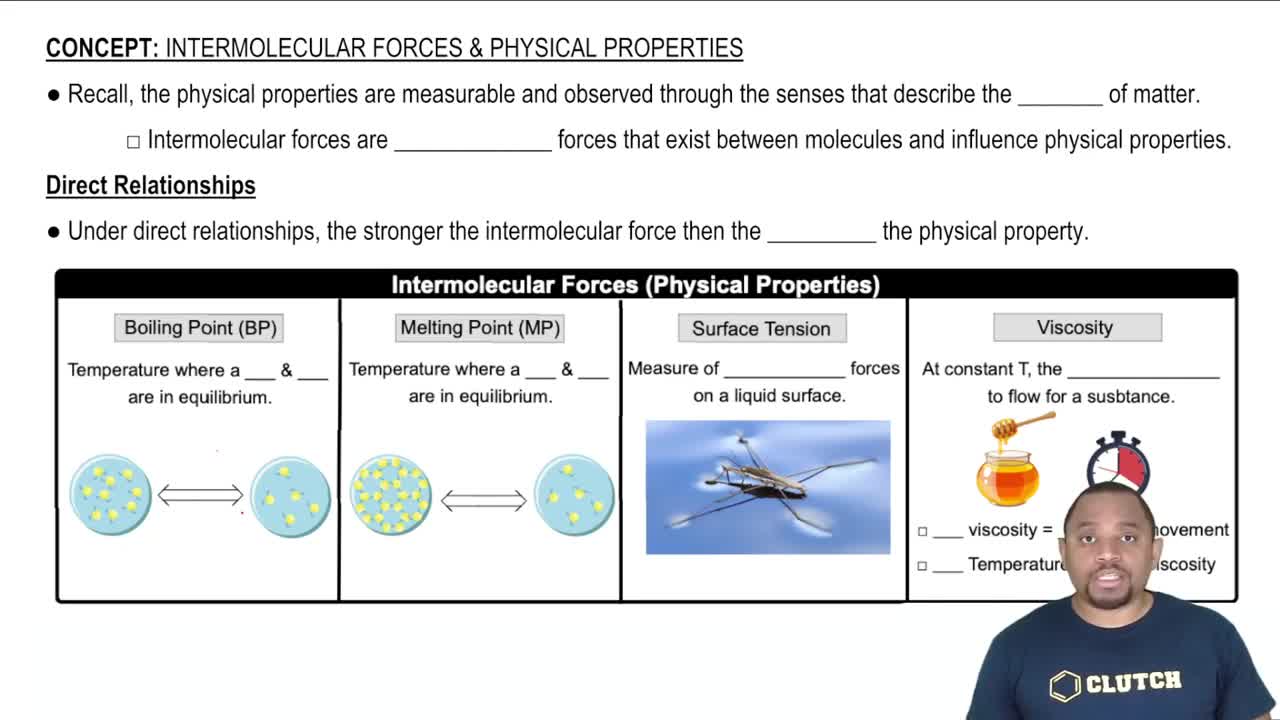
Viscosity
Viscosity is a measure of a fluid's resistance to flow. It indicates how thick or thin a liquid is, affecting how easily it moves. In general, higher viscosity means a thicker fluid that flows more slowly, while lower viscosity indicates a thinner fluid that flows more easily. Understanding viscosity is crucial for evaluating the performance of motor oils under different temperature conditions.
Recommended video:
Intermolecular Forces and Properties
Multigrade vs. Single-Grade Oils
Multigrade oils are formulated to perform well at a range of temperatures, typically indicated by two viscosity ratings (e.g., 10W-30). In contrast, single-grade oils have a fixed viscosity rating (e.g., 30W) and are designed for specific temperature ranges. The additives in multigrade oils help maintain a more stable viscosity across varying temperatures, making them less temperature-dependent than single-grade oils.
Recommended video:
Temperature Dependence of Viscosity
The viscosity of fluids generally decreases as temperature increases, meaning they flow more easily when heated. Single-grade oils experience significant changes in viscosity with temperature fluctuations, which can affect engine performance. Multigrade oils, however, are engineered with viscosity index improvers that minimize these changes, allowing them to maintain consistent performance across a broader temperature range.
Recommended video:
Kw Temperature Dependence