Phosgene, COCl2(g), is a toxic gas used as an agent of warfare in World War I. (b) Using the table of bond dissociation energies (Table 9.3) and the value ΔH°f = 716.7 kJ/mol for C(g), estimate ΔH°f for COCl2(g) at 25 °C. Compare your answer to the actual ΔH°f given in Appendix B, and explain why your calculation is only an estimate.
Ch.9 - Thermochemistry: Chemical Energy
Chapter 9, Problem 152b
Acid spills are often neutralized with sodium carbonate or sodium hydrogen carbonate. For neutralization of acetic acid, the unbalanced equations are
(1) CH3CO2H(l) + Na2CO3(s) → CH3CO2Na(aq) + CO2(g) + H2O(l)
(2) CH3CO2H(l) + NaHCO3(s) → CH3CO2Na(aq) + CO2(g) + H2O(l)
(b) How many kilograms of each substance is needed to neutralize a 1.000-gallon spill of pure acetic acid (density = 1.049 g/mL)?

Verified Solution
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Neutralization Reaction
A neutralization reaction occurs when an acid reacts with a base to produce water and a salt. In this context, acetic acid (a weak acid) reacts with sodium carbonate or sodium bicarbonate (bases) to form sodium acetate, carbon dioxide, and water. Understanding this reaction is crucial for determining the stoichiometry involved in neutralizing the acid spill.
Recommended video:
Guided course

Lewis Dot Structures: Neutral Compounds
Stoichiometry
Stoichiometry is the calculation of reactants and products in chemical reactions based on balanced equations. It allows us to determine the exact amounts of substances needed for a reaction. In this case, stoichiometry will help calculate how many kilograms of sodium carbonate or sodium bicarbonate are required to neutralize a specific volume of acetic acid.
Recommended video:
Guided course

Stoichiometry Concept
Density and Volume Conversion
Density is the mass per unit volume of a substance, and it is essential for converting between volume and mass. Given the density of acetic acid, we can convert the volume of the spill (1.000 gallon) into grams, which is necessary for calculating the amount of base needed for neutralization. This conversion is a key step in solving the problem.
Recommended video:
Guided course
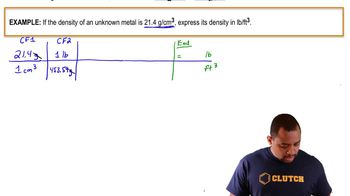
Density Conversion Example
Related Practice
Textbook Question
682
views
Textbook Question
Acid spills are often neutralized with sodium carbonate or sodium hydrogen carbonate. For neutralization of acetic acid, the unbalanced equations are
112 CH3CO2H1l2 + Na2CO31s2 S
CH3CO2Na1aq2 + CO21g2 + H2O1l2 122 CH3CO2H1l2 + NaHCO31s2
CH3CO2Na1aq2 + CO21g2 + H2O1l2
(c) How much heat in kilojoules is absorbed or liberated in each reaction? See Appendix B for standard heats of for- mation; ΔH°f = - 726.1 kJ>mol for CH3CO2 Na(aq).
644
views
Textbook Question
Acid spills are often neutralized with sodium carbonate or sodium hydrogen carbonate. For neutralization of acetic acid, the unbalanced equations are
(1) CH3CO2H(l) + Na2CO3(s) → CH3CO2Na(aq) + CO2(g) + H2O(l)
(2) CH3CO2H(l) + NaHCO3(s) → CH3CO2Na(aq) + CO2(g) + H2O(l)
(a) Balance both equations.
686
views
Textbook Question
(a) Write a balanced equation for the reaction of potassium metal with water.
1359
views
Textbook Question
(b) Use the data in Appendix B to calculate ΔH° for the reaction of potassium metal with water.
1135
views
Textbook Question
(c) Assume that a chunk of potassium weighing 7.55 g is dropped into 400.0 g of water at 25.0 °C. What is the final temperature of the water if all the heat released is used to warm the water?
390
views
