Textbook Question
What is the oxidation state of the metal in each of the complexes?
a. AgCl2–
b. [Cr(H2O)5Cl]2+
c. [Co(NCS)4]2–
d. [ZrF8]4–
e. [Fe(EDTA)(H2O)]–
89
views
 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.78a
Problem 21.78a


What is the oxidation state of the metal in each of the complexes?
a. AgCl2–
b. [Cr(H2O)5Cl]2+
c. [Co(NCS)4]2–
d. [ZrF8]4–
e. [Fe(EDTA)(H2O)]–
What is the oxidation state of the metal in each of the complexes?
a. [Ni(CN)5]3–
b. Ni(CO)4
c. [Co(en)2(H2O)Br]2+
d. [Cu(H2O)2(C2O4)2]2–
e. Co(NH3)3(NO2)3
What role does EDTA4- play as a trace additive to mayonnaise? Would the glycinate ion (H2NCH2CH2NH2) be an effective substitute for EDTA4-?
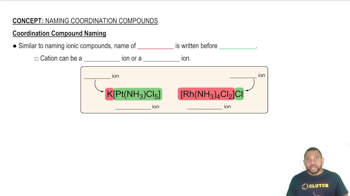
What is the systematic name for each of the following ions?
(c) [Co(CO3)3]3-
(d) [Pt(en)2(SCN)2]2+
Assign a systematic name to each of the following ions.
(a) [AuCl4]-
(b) [Fe(CN)6]4-
Assign a systematic name to each of the following ions.
(c) [Fe(H2O)5NCS]2+
(d) [Cr(NH3)2(C2O4)2]-