What is the systematic name for each of the following ions?
(a) [MnCl4]2-
(b) [Ni(NH3)6]2+
 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.79b
Problem 21.79b
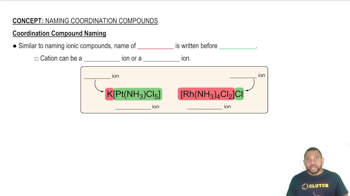


What is the systematic name for each of the following ions?
(a) [MnCl4]2-
(b) [Ni(NH3)6]2+
What is the systematic name for each of the following ions?
(c) [Co(CO3)3]3-
(d) [Pt(en)2(SCN)2]2+
Assign a systematic name to each of the following ions.
(a) [AuCl4]-
(b) [Fe(CN)6]4-
Based on the wavelength of maximum absorption of the cobalt complexes, arrange the following ligands in a spectrochemical series from weakest-field to strongest-field ligand.
(a) Cl- < NCS- < H2O < NH3
(b) Cl- < NCS- < H2O < NH3
(c) H2O < Cl- < NH3 < NCS-
(d) Cl- < H2O < NCS- < NH3
What is the systematic name for each of the following coordination compounds?
(c) [Co(NH3)4Br2]Br
(d) Cu(gly)2
What is the systematic name for each of the following coordination compounds?
(a) Cs[FeCl4]
(b) [V(H2O)6](NO3)3