Here are the essential concepts you must grasp in order to answer the question correctly.
Metallic Bonding
Metallic bonding occurs in metals, where positively charged metal ions are surrounded by a 'sea' of delocalized electrons. This arrangement allows metals to conduct electricity and heat, and gives them malleability and ductility. The relatively low melting point of sodium metal (97.8 °C) indicates that the metallic bonds in sodium are weaker compared to ionic bonds.
Recommended video:
Ionic Bonding
Ionic bonding is the electrostatic attraction between positively charged cations and negatively charged anions, typically formed when metals transfer electrons to nonmetals. This type of bond results in the formation of a crystalline lattice structure, which is responsible for the high melting points of ionic compounds. Sodium chloride's high melting point (801 °C) reflects the strong ionic bonds that hold the lattice together.
Recommended video:
Melting Point as a Measure of Bond Strength
The melting point of a substance is a key indicator of the strength of the bonds holding its particles together. Generally, higher melting points suggest stronger intermolecular or interionic forces. In this case, the significant difference in melting points between sodium and sodium chloride suggests that ionic bonds are much stronger than metallic bonds, as evidenced by the much higher energy required to break the ionic bonds in sodium chloride.
Recommended video:
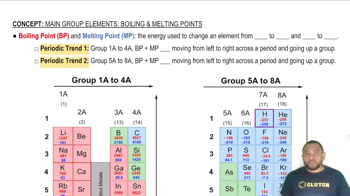
Boiling Point and Melting Point