Here are the essential concepts you must grasp in order to answer the question correctly.
Covalent Bonding
Covalent bonding occurs when two atoms share electrons to achieve a full outer shell, leading to the formation of molecules. In the case of CO2, carbon shares electrons with two oxygen atoms, resulting in a linear molecular structure. This type of bonding is characterized by the specific arrangement of atoms and the presence of distinct molecular properties.
Recommended video:
Network Covalent Solids
Network covalent solids, like SiO2, consist of a continuous network of covalent bonds extending throughout the material. In SiO2, each silicon atom is bonded to four oxygen atoms in a tetrahedral arrangement, creating a rigid three-dimensional structure. This results in high melting points and hardness, distinguishing them from molecular compounds.
Recommended video:
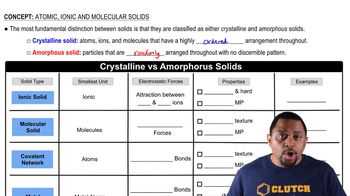
Crystalline vs Amorphous Solids
Differences in Elemental Properties
The differences in bonding between carbon and silicon with oxygen stem from their positions in the periodic table. Carbon, a nonmetal, forms discrete molecular compounds like CO2, while silicon, a metalloid, tends to form extensive covalent networks. This variance in bonding behavior is influenced by factors such as electronegativity and atomic size, affecting how these elements interact with oxygen.
Recommended video:
Elemental Forms of Elements
 Verified step by step guidance
Verified step by step guidance