Here are the essential concepts you must grasp in order to answer the question correctly.
Covalent Network Solids
Covalent network solids are materials where atoms are bonded together by a network of covalent bonds, forming a continuous structure. This results in high melting points, hardness, and electrical non-conductivity. Silicon carbide (SiC) exemplifies this type of solid, as its atoms are arranged in a three-dimensional lattice similar to that of diamond, contributing to its exceptional strength and thermal stability.
Recommended video:
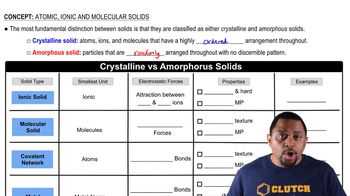
Crystalline vs Amorphous Solids
Crystal Structure
The crystal structure of a material refers to the orderly arrangement of atoms within the solid. In the case of silicon carbide, the structure can be visualized as a repeating pattern of silicon and carbon atoms, forming tetrahedral units. Understanding the crystal structure is essential for predicting the material's properties, such as its hardness and thermal conductivity.
Recommended video:
Tetrahedral Coordination
Tetrahedral coordination is a geometric arrangement where a central atom is surrounded by four other atoms at the corners of a tetrahedron. In SiC, silicon atoms are tetrahedrally coordinated with carbon atoms, leading to a strong and stable structure. This coordination is crucial for the material's mechanical properties and contributes to its high resistance to thermal and chemical degradation.
Recommended video:
Molecular Geometry of Coordination Complexes