Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas through the equation PV = nRT. This law is essential for understanding how gas behaves under different conditions. In this scenario, it helps determine the volume of gasoline vapor that can be produced from the remaining gasoline in the tank, which is crucial for calculating the distance the vehicle can travel.
Recommended video:
Density and Volume Conversion
Density is defined as mass per unit volume and is crucial for converting between mass and volume. In this question, the density of liquid gasoline (0.75 g/mL) allows us to convert the mass of gasoline vapor into a volume that can be used in calculations. Understanding how to manipulate these units is key to finding the total volume of gasoline vapor available for combustion.
Recommended video:
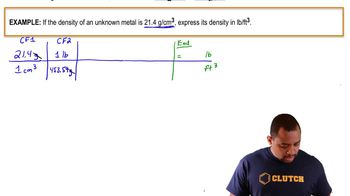
Density Conversion Example
Molar Mass and Stoichiometry
Molar mass is the mass of one mole of a substance, which is vital for converting between grams and moles. In this case, the average molar mass of gasoline (105 g/mol) is used to determine how many moles of gasoline vapor are present. This information is necessary for calculating the total energy available for combustion and, ultimately, the distance the vehicle can travel based on its fuel efficiency.
Recommended video: