Here are the essential concepts you must grasp in order to answer the question correctly.
Osmotic Pressure
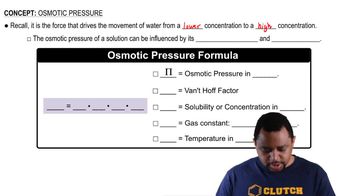
Osmotic pressure is the pressure required to prevent the flow of solvent into a solution through a semipermeable membrane. It is directly proportional to the concentration of solute particles in the solution and can be calculated using the formula π = iCRT, where π is the osmotic pressure, i is the van 't Hoff factor, C is the molarity of the solution, R is the ideal gas constant, and T is the temperature in Kelvin.
Recommended video:
Molar Mass
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). It is calculated by summing the atomic masses of all the atoms in a molecule. In this context, determining the molar mass of hemoglobin involves using the mass of the solute and the volume of the solution to find the number of moles present, which can then be used to calculate the molar mass.
Recommended video:
Ideal Solution Assumption
The ideal solution assumption posits that solute-solvent interactions are similar to solute-solute and solvent-solvent interactions, leading to predictable behavior in solutions. In this problem, it is assumed that hemoglobin does not dissociate in water, allowing for the use of the osmotic pressure equation without accounting for any changes in particle number due to dissociation, simplifying the calculation of molar mass.
Recommended video: