Consider the equilibrium B1aq2 + H2O1l2 Δ HB+1aq2 + OH-1aq2. Suppose that a salt of HB+1aq2 is added to a solution of B1aq2 at equilibrium. (c) Will the pH of the solution increase, decrease, or stay the same?
(a) Calculate the percent ionization of 0.125 M lactic acid 1Ka = 1.4 * 10-42.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Ionization of Acids
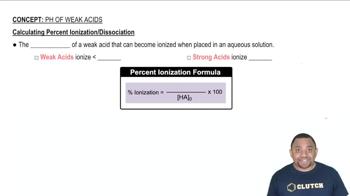
Percent Ionization
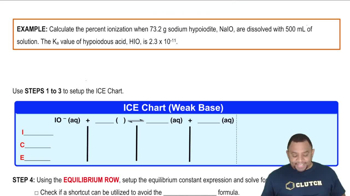
Equilibrium Constant (Ka)

a. Calculate the percent ionization of 0.007 M butanoic acid (𝐾𝑎=1.5×10−5).
(b) Calculate the percent ionization of 0.0075 M butanoic acid in a solution containing 0.085 M sodium butanoate.
Which of the following solutions is a buffer? (a) A solution made by mixing 100 mL of 0.100 M CH3COOH and 50 mL of 0.100 M NaOH, (b) a solution made by mixing 100 mL of 0.100 M CH3COOH and 500 mL of 0.100 M NaOH, (c) A solution made by mixing 100 mL of 0.100 M CH3COOH and 50 mL of 0.100 M HCl, (d) A solution made by mixing 100 mL of 0.100 M CH3COOK and 50 mL of 0.100 M KCl.
(a) Calculate the pH of a buffer that is 0.12 M in lactic acid and 0.11 M in sodium lactate.
You are asked to prepare a pH = 3.00 buffer solution starting from 1.25 L of a 1.00 M solution of hydrofluoric acid (HF) and any amount you need of sodium fluoride (NaF). (a) What is the pH of the hydrofluoric acid solution prior to adding sodium fluoride?
