Here are the essential concepts you must grasp in order to answer the question correctly.
Ionization of Weak Acids
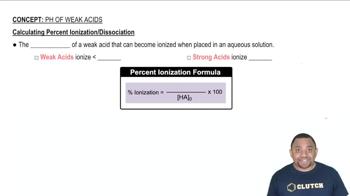
Weak acids, like butanoic acid, do not completely dissociate in solution. Instead, they establish an equilibrium between the undissociated acid and its ions. The degree to which a weak acid ionizes can be quantified, and this is crucial for calculating percent ionization, which indicates how much of the acid has converted to ions.
Recommended video:
Calculating Percent Ionization of Weak Acids
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation relates the pH of a buffer solution to the concentration of the acid and its conjugate base. In this case, butanoic acid and sodium butanoate form a buffer system. This equation is essential for determining the pH, which is necessary for calculating the percent ionization of the weak acid in the presence of its salt.
Recommended video:
Henderson-Hasselbalch Equation
Percent Ionization Calculation
Percent ionization is calculated by taking the concentration of ionized acid at equilibrium, dividing it by the initial concentration of the acid, and multiplying by 100. This metric provides insight into the strength of the acid and its behavior in solution, especially when influenced by the presence of a conjugate base, as seen with sodium butanoate.
Recommended video:
Calculating Percent Ionization of Weak Acids