Textbook Question
Consider the equilibrium B(aq) + H2O(l) ⇌ HB+(aq) + OH–(aq). Suppose that a salt of HB+(aq) is added to a solution of B(aq) at equilibrium. (c) Will the pH of the solution increase, decrease, or stay the same?
472
views
 Verified step by step guidance
Verified step by step guidance

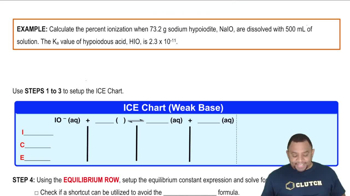
Consider the equilibrium B(aq) + H2O(l) ⇌ HB+(aq) + OH–(aq). Suppose that a salt of HB+(aq) is added to a solution of B(aq) at equilibrium. (c) Will the pH of the solution increase, decrease, or stay the same?
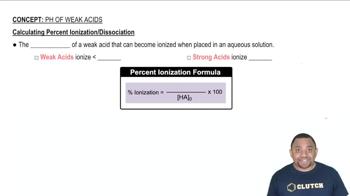
(b) Calculate the percent ionization of 0.0075 M butanoic acid in a solution containing 0.085 M sodium butanoate.
(a) Calculate the percent ionization of 0.125 M lactic acid (Ka = 1.4 × 10-4).
(b) Calculate the percent ionization of 0.125 M lactic acid in a solution containing 0.0075 M sodium lactate.