The figure that follows contains ball-and-stick drawings of three possible shapes of an AF4 molecule. (a) For each shape, give the electron-domain geometry on which the molecular geometry is based. i.
For each of these contour representations of molecular orbitals, identify (c) whether the MO is bonding or antibonding (i)

 Verified step by step guidance
Verified step by step guidance
Verified Solution
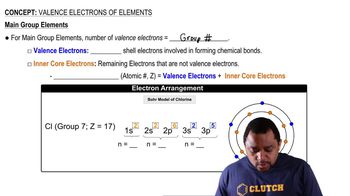
Key Concepts
Molecular Orbitals (MOs)

Bonding vs. Antibonding Orbitals

Contour Representations
The figure that follows contains ball-and-stick drawings of three possible shapes of an AF4 molecule. (a) For each shape, give the electron-domain geometry on which the molecular geometry is based. iii.
For each of these contour representations of molecular orbitals, identify (a) the atomic orbitals (s or p) used to construct the MO (i)
For each of these contour representations of molecular orbitals, identify (a) the atomic orbitals (s or p) used to construct the MO (iii)
For each of these contour representations of molecular orbitals, identify (b) the type of MO (s or p) (iii)
Consider the Lewis structure for acetic acid, which is known as vinegar: (b) What are the hybridizations of the orbitals on the two oxygen atoms, and what are the approximate bond angles at the oxygen that is connected to carbon and hydrogen? What are the hybridizations of the orbitals on the two oxygen atoms?
