The following is part of a molecular orbital energy-level diagram for MOs constructed from 1s atomic orbitals.
(a) What labels do we use for the two MOs shown?

 Verified step by step guidance
Verified step by step guidance


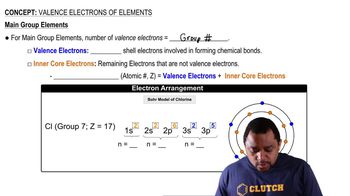
The following is part of a molecular orbital energy-level diagram for MOs constructed from 1s atomic orbitals.
(a) What labels do we use for the two MOs shown?
For each of these contour representations of molecular orbitals, identify (a) the atomic orbitals (s or p) used to construct the MO (i)
For each of these contour representations of molecular orbitals, identify (a) the atomic orbitals (s or p) used to construct the MO (iii)
For each of these contour representations of molecular orbitals, identify (b) the type of MO (s or p) (i)
For each of these contour representations of molecular orbitals, identify (c) whether the MO is bonding or antibonding (i)
(a) An AB2 molecule is linear. How many nonbonding electron pairs are around the A atom from this information?