Figure 7.4 shows the radial probability distribution functions for the 2s orbitals and 2p orbitals. (a) Which orbital, 2s or 2p, has more electron density close to the nucleus?
(b) Repeat these calculations using Slater’s rules.

Verified Solution
Key Concepts
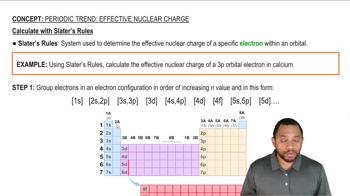
Slater's Rules
Effective Nuclear Charge (Z_eff)
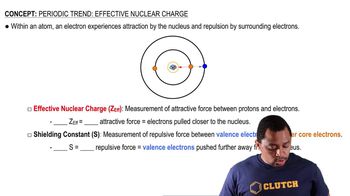
Shielding Effect

Figure 7.4 shows the radial probability distribution functions for the 2s orbitals and 2p orbitals. (b) How would you modify Slater's rules to adjust for the difference in electronic penetration of the nucleus for the 2s and 2p orbitals?
(a) If the core electrons were totally effective at screening the valence electrons and the valence electrons provided no screening for each other, what would be the effective nuclear charge acting on the 3s and 3p valence electrons in P?
(d) If you remove a single electron from a P atom, which orbital will it come from?
The As ¬ As bond length in elemental arsenic is 2.48 Å. The Cl ¬ Cl bond length in Cl2 is 1.99 Å. (a) Based on these data, what is the predicted As ¬ Cl bond length in arsenic trichlo- ride, AsCl3, in which each of the three Cl atoms is bonded to the As atom?
