Consider the stable elements through lead (Z = 82). In how many instances are the atomic weights of the elements out of order relative to the atomic numbers of the elements?
Ch.7 - Periodic Properties of the Elements
Chapter 7, Problem 81a
(a) If the core electrons were totally effective at screening the valence electrons and the valence electrons provided no screening for each other, what would be the effective nuclear charge acting on the 3s and 3p valence electrons in P?
 Verified step by step guidance
Verified step by step guidance1
insert step 1> Identify the atomic number of phosphorus (P), which is 15. This represents the total number of protons in the nucleus.
insert step 2> Determine the number of core electrons in phosphorus. Phosphorus has an electron configuration of 1s^2 2s^2 2p^6 3s^2 3p^3. The core electrons are those in the 1s, 2s, and 2p orbitals, totaling 10 electrons.
insert step 3> Calculate the effective nuclear charge (Z_eff) using the formula: Z_eff = Z - S, where Z is the atomic number and S is the number of core electrons.
insert step 4> Substitute the values into the formula: Z_eff = 15 (atomic number of P) - 10 (core electrons).
insert step 5> The result from the calculation in step 4 gives the effective nuclear charge experienced by the 3s and 3p valence electrons, assuming no screening by the valence electrons themselves.
Recommended similar problem, with video answer:

Verified Solution
This video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Effective Nuclear Charge (Z_eff)
Effective nuclear charge (Z_eff) is the net positive charge experienced by an electron in a multi-electron atom. It accounts for the actual nuclear charge (the number of protons) minus the shielding effect of inner (core) electrons. In the case of phosphorus (P), Z_eff helps determine how strongly the valence electrons are attracted to the nucleus, influencing their energy levels and chemical behavior.
Recommended video:
Guided course
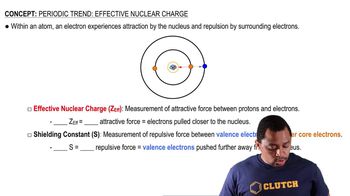
Effective Nuclear Charge
Electron Shielding
Electron shielding occurs when inner electrons partially block the attraction between the nucleus and the outer (valence) electrons. This effect reduces the effective nuclear charge felt by the valence electrons. In the context of the question, if core electrons are fully effective at screening, the valence electrons would experience a significantly lower Z_eff, impacting their energy and reactivity.
Recommended video:
Guided course

Electron Geometry
Valence Electrons
Valence electrons are the outermost electrons of an atom and are primarily responsible for its chemical properties and bonding behavior. In phosphorus, the 3s and 3p orbitals contain the valence electrons. Understanding their interaction with the nucleus and the effect of shielding is crucial for predicting how phosphorus will react with other elements.
Recommended video:
Guided course

Transition Metals Valence Electrons
Related Practice
Textbook Question
483
views
Textbook Question
Figure 7.4 shows the radial probability distribution functions for the 2s orbitals and 2p orbitals. (a) Which orbital, 2s or 2p, has more electron density close to the nucleus?
843
views
Textbook Question
Figure 7.4 shows the radial probability distribution functions for the 2s orbitals and 2p orbitals. (b) How would you modify Slater's rules to adjust for the difference in electronic penetration of the nucleus for the 2s and 2p orbitals?
2114
views
Textbook Question
(b) Repeat these calculations using Slater’s rules.
996
views
Textbook Question
(c) Detailed calculations indicate that the effective nuclear charge is 5.6+ for the 3s electrons and 4.9+ for the 3p electrons. Why are the values for the 3s and 3p electrons different?
Textbook Question
(d) If you remove a single electron from a P atom, which orbital will it come from?
1056
views
