Which of the following represent impossible combinations of n and l? (a) 1p (b) 4s (c) 5f (d) 2d
Ch.6 - Electronic Structure of Atoms
Chapter 6, Problem 64c
Sketch the shape and orientation of the following types of orbitals: (c) dx2 - y2.
 Verified step by step guidance
Verified step by step guidance1
Understand that the d orbitals are a set of five orbitals in the third energy level and higher, each with a unique shape and orientation.
Recognize that the \(d_{x^2 - y^2}\) orbital is one of these five d orbitals, characterized by its distinct shape.
Visualize the \(d_{x^2 - y^2}\) orbital as having four lobes oriented along the x and y axes, forming a cloverleaf shape.
Note that the lobes of the \(d_{x^2 - y^2}\) orbital lie in the xy-plane, with the nodal planes along the x = 0 and y = 0 lines, meaning there is no electron density along these lines.
Remember that the orientation of the \(d_{x^2 - y^2}\) orbital is crucial for understanding its role in chemical bonding, particularly in transition metal complexes.

Verified Solution
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Atomic Orbitals
Atomic orbitals are regions in an atom where there is a high probability of finding electrons. They are defined by quantum numbers and have distinct shapes, such as s, p, d, and f orbitals. Each type of orbital has a specific geometric configuration that influences the chemical properties of the atom.
Recommended video:
Guided course

Atomic Orbitals Example
d-Orbitals
d-orbitals are a set of five orbitals that are more complex in shape compared to s and p orbitals. They are important for transition metals and can hold a maximum of ten electrons. The dx2 - y2 orbital, in particular, has a unique shape that resembles a four-leaf clover, oriented along the x and y axes.
Recommended video:
Guided course
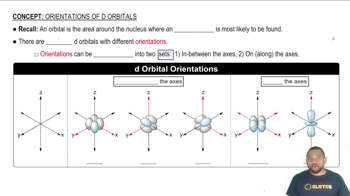
d Orbital Orientations
Orbital Orientation
Orbital orientation refers to the spatial arrangement of orbitals in relation to the axes of a coordinate system. For the dx2 - y2 orbital, its lobes are oriented along the x and y axes, with nodal planes along the diagonals. Understanding this orientation is crucial for predicting molecular geometry and bonding behavior in chemistry.
Recommended video:
Guided course

d Orbital Orientations
Related Practice
Textbook Question
1080
views
Textbook Question
For the table that follows, write which orbital goes with the quantum numbers. Don't worry about x, y, z subscripts. If the quantum numbers are not allowed, write 'not allowed.' n l ml Orbital 2 1 -1 2p (example) 1 0 0 3 -3 2 3 2 -2 2 0 -1 0 0 0 4 2 1 5 3 0
1056
views
Textbook Question
Sketch the shape and orientation of the following types of orbitals: (a) s.
1177
views
Textbook Question
(c) What can you say about the average distance from the nucleus of an electron in a 2s orbital as compared with a 3s orbital?
677
views
Textbook Question
(d) For the hydrogen atom, list the following orbitals in order of increasing energy (that is, most stable ones first): 4f, 6s, 3d, 1s, 2p.
859
views
Textbook Question
(a) With reference to Figure 6.19, what is the relationship between the number of nodes in an s orbital and the value of the principal quantum number?
784
views
