Here are the essential concepts you must grasp in order to answer the question correctly.
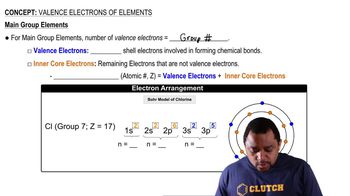
Valence Electrons
Valence electrons are the outermost electrons of an atom and are crucial for determining how an element will react chemically. For fluorine, which has an atomic number of 9, there are 7 valence electrons in its outer shell (2s² 2p⁵). These electrons are involved in bonding and influence the element's reactivity.
Recommended video:
Transition Metals Valence Electrons
Core Electrons
Core electrons are the electrons that are not involved in bonding and are located in the inner shells of an atom. In the case of fluorine, there are 2 core electrons in the 1s orbital. Understanding the distinction between core and valence electrons is essential for predicting chemical behavior and stability.
Recommended video:
Main Group Elements Valence Electrons
Unpaired Electrons
Unpaired electrons are those that occupy an orbital alone and are significant in determining the magnetic properties and reactivity of an atom. For fluorine, there are 1 unpaired electron in the 2p orbital, which contributes to its high reactivity as it seeks to pair with another electron to achieve a stable octet configuration.
Recommended video:
 Verified step by step guidance
Verified step by step guidance