Here are the essential concepts you must grasp in order to answer the question correctly.
Core Electrons
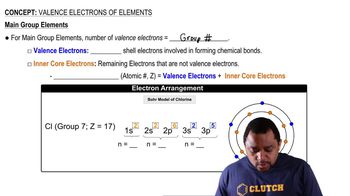
Core electrons are the electrons in an atom that are not involved in chemical bonding and are located in the inner shells, closest to the nucleus. They are typically shielded from the effects of external electric fields and do not participate in reactions, unlike valence electrons, which are found in the outermost shell and are responsible for an atom's chemical properties.
Recommended video:
Main Group Elements Valence Electrons
Electron Configuration
Electron configuration describes the distribution of electrons in an atom's atomic orbitals. It is represented using a notation that indicates the energy levels and sublevels occupied by electrons. Understanding electron configuration is essential for identifying core and valence electrons, as it helps visualize which electrons are involved in bonding and which are core electrons.
Recommended video:
Electron Configuration Example
Shielding Effect
The shielding effect refers to the phenomenon where inner electrons (core electrons) reduce the effective nuclear charge felt by outer electrons (valence electrons). This effect influences the chemical behavior of an atom, as it affects the attraction between the nucleus and the valence electrons, thereby impacting ionization energy and reactivity.
Recommended video: