Here are the essential concepts you must grasp in order to answer the question correctly.
Valence Electrons
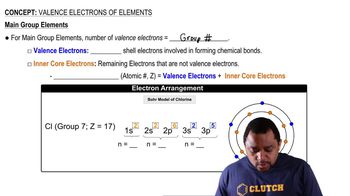
Valence electrons are the outermost electrons of an atom and are crucial for determining how an element will react chemically. For sodium (Na), which has an atomic number of 11, there is one valence electron in its outermost shell (3s1). This single valence electron plays a significant role in sodium's reactivity, particularly in forming ionic bonds.
Recommended video:
Transition Metals Valence Electrons
Core Electrons
Core electrons are the electrons that are not involved in chemical bonding and are located in the inner shells of an atom. In sodium, there are 10 core electrons, which are found in the first two energy levels (1s2 and 2s2 2p6). These electrons shield the valence electron from the full effect of the nucleus's positive charge, influencing the atom's overall stability.
Recommended video:
Main Group Elements Valence Electrons
Unpaired Electrons
Unpaired electrons are those that occupy an orbital alone rather than in pairs, and they are significant in determining the magnetic properties and reactivity of an atom. In the case of sodium, there is one unpaired electron in the 3s orbital. This unpaired electron is responsible for sodium's tendency to lose an electron and form a positive ion (Na+), which is a key aspect of its chemical behavior.
Recommended video:
 Verified step by step guidance
Verified step by step guidance