Molybdenum metal must absorb radiation with an energy higher than 7.22 * 10-19 J ('energy threshold') before it can eject an electron from its surface via the photoelectric effect. (b) What wavelength of radiation will provide a photon of this energy?
Does the hydrogen atom 'expand' or 'contract' when an electron is excited from the n = 1 state to the n = 3 state?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Quantum States
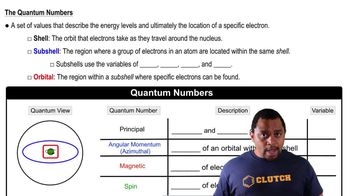
Electron Excitation

Atomic Radius

Molybdenum metal must absorb radiation with an energy higher than 7.22 * 10-19 J ('energy threshold') before it can eject an electron from its surface via the photoelectric effect. (c) If molybdenum is irradiated with light of wavelength of 240 nm, what is the maximum possible velocity of the emitted electrons?
Classify each of the following statements as either true or false: (a) A hydrogen atom in the n = 3 state can emit light at only two specific wavelengths (b) a hydrogen atom in the n = 2 state is at a lower energy than one in the n = 1 state (c) the energy of an emitted photon equals the energy difference of the two states involved in the emission.
Is energy emitted or absorbed when the following electronic transitions occur in hydrogen? (a) from n = 3 to n = 2 (c) an electron adds to the H+ ion and ends up in the n = 2 shell?
Is energy emitted or absorbed when the following electronic transitions occur in hydrogen? (b) from an orbit of radius 0.846 nm to one of radius 0.212 nm
