(b) How many molecules of C13H18O2 are in this tablet?
Very small semiconductor crystals, composed of approximately 1000 to 10,000 atoms, are called quantum dots. Quantum dots made of the semiconductor CdSe are now being used in electronic reader and tablet displays because they emit light efficiently and in multiple colors, depending on dot size. The density of CdSe is 5.82 g/cm3. (b) CdSe quantum dots that are 2.5 nm in diameter emit blue light upon stimulation. Assuming that the dot is a perfect sphere and that the empty space in the dot can be neglected, calculate how many Cd atoms are in one quantum dot of this size.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Quantum Dots
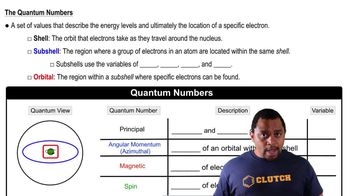
Volume and Density Calculations

Atomic Composition and Molar Mass

"The diameter of a rubidium atom is 495 pm We will consider two different ways of placing the atoms on a surface. In arrangement A, all the atoms are lined up with one another to form a square grid. Arrangement B is called a close-packed arrangement because the atoms sit in the 'depressions' formed by the previous row of atoms:
(c) By what factor has the number of atoms on the surface increased in going to arrangement B from arrangement A?
"The diameter of a rubidium atom is 495 pm We will consider two different ways of placing the atoms on a surface. In arrangement A, all the atoms are lined up with one another to form a square grid. Arrangement B is called a close-packed arrangement because the atoms sit in the 'depressions' formed by the previous row of atoms:
(c) If extended to three dimensions, which arrangement would lead to a greater density for Rb metal?"
(a) Assuming the dimensions of the nucleus and atom shown in Figure 2.10, what fraction of the volume of the atom is taken up by the nucleus?
(b) Using the mass of the proton from Table 2.1 and assuming its diameter is 1.0 * 10-15 m, calculate the density of a proton in g>cm3.
Identify the element represented by each of the following symbols and give the number of protons and neutrons in each: (a) 7433X
