A concentration of 10–100 parts per billion (by mass) of Ag+ is an effective disinfectant in swimming pools. However, if the concentration exceeds this range, the Ag+ can cause adverse health effects. One way to maintain an appropriate concentration of Ag+ is to add a slightly soluble salt to the pool. Using Ksp values from Appendix D, calculate the equilibrium concentration of Ag+ in parts per billion that would exist in equilibrium with (b) AgBr (c) AgI.
Baking soda (sodium bicarbonate, NaHCO3) reacts with acids in foods to form carbonic acid (H2CO3), which in turn decomposes to water and carbon dioxide gas. In a cake batter, the CO2(g) forms bubbles and causes the cake to rise. (b) The density of baking soda is 2.16 g/cm3. Calculate the concentration of lactic acid in one cup of sour milk (assuming the rule of thumb applies), in units of mol/L. (One cup = 236.6 mL = 48 teaspoons).
Recommended similar problem, with video answer:

Verified Solution
Key Concepts
Acid-Base Reactions

Concentration and Molarity

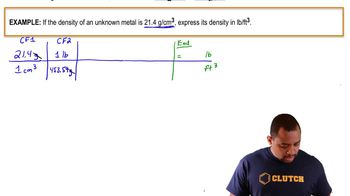
Density and Volume Conversion
Fluoridation of drinking water is employed in many places to aid in the prevention of tooth decay. Typically. the Fion concentration is adjusted to about 1 ppm. Some water supplies are also 'hard'; that is, they contain certain cations such as Ca2 + that interfere with the action of soap. Consider a case where the concentration of Ca2 + is 8 ppm. Could a precipitate of CaF2 form under these conditions? (Make any necessary approximations.)
Baking soda (sodium bicarbonate, NaHCO3) reacts with acids in foods to form carbonic acid (H2CO3), which in turn decomposes to water and carbon dioxide gas. In a cake batter, the CO2(g) forms bubbles and causes the cake to rise. (a) A rule of thumb in baking is that 1/2 teaspoon of baking soda is neutralized by one cup of sour milk. The acid component in sour milk is lactic acid, CH3CH(OH)COOH. Write the chemical equation for this neutralization reaction.
Baking soda (sodium bicarbonate, NaHCO3) reacts with acids in foods to form carbonic acid (H2CO3), which in turn decomposes to water and carbon dioxide gas. In a cake batter, the CO2(g) forms bubbles and causes the cake to rise. (c) If 1/2 teaspoon of baking soda is indeed completely neutralized by the lactic acid in sour milk, calculate the volume of carbon dioxide gas that would be produced at 1 atm pressure, in an oven set to 350 F.
In nonaqueous solvents, it is possible to react HF to create H2F+. Which of these statements follows from this observation? (a) HF can act like a strong acid in nonaqueous solvents, (b) HF can act like a base in nonaqueous solvents, (c) HF is thermodynamically unstable, (d) There is an acid in the nonaqueous medium that is a stronger acid than HF.
