Here are the essential concepts you must grasp in order to answer the question correctly.
pH Scale
The pH scale measures the acidity or basicity of a solution, ranging from 0 to 14. A pH of 7 is neutral, below 7 is acidic, and above 7 is basic. Each unit change in pH represents a tenfold change in hydrogen ion concentration, meaning that a decrease in pH indicates an increase in acidity.
Recommended video:
Hydrogen Ion Concentration
Hydrogen ion concentration, denoted as [H+], is a measure of the amount of hydrogen ions present in a solution. It is directly related to pH, where a lower pH corresponds to a higher concentration of H+. For example, a change of 2.00 units in pH results in a change in [H+] by a factor of 10^2, or 100 times.
Recommended video:
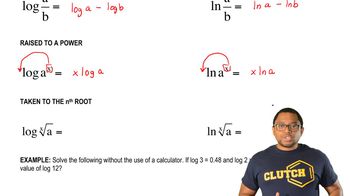
Logarithmic Relationship
The relationship between pH and hydrogen ion concentration is logarithmic, meaning that pH is the negative logarithm of the hydrogen ion concentration: pH = -log[H+]. This implies that small changes in pH can lead to significant changes in [H+], which is crucial for understanding how acidity affects chemical reactions and biological processes.
Recommended video:
 Verified step by step guidance
Verified step by step guidance