At 373 K, 𝐾𝑝 = 0.416 for the equilibrium 2 NOBr(𝑔) ⇌ 2 NO(𝑔) + Br2(𝑔) If the pressures of NOBr(𝑔) and NO(𝑔) are equal, what is the equilibrium pressure of Br2(𝑔)?
At 80°C, 𝐾𝑐 = 1.87×10−3 for the reaction PH3BCl3(𝑠) ⇌ PH3(𝑔) + BCl3(𝑔) (a) Calculate the equilibrium concentrations of PH3 and BCl3 if a solid sample of PH3BCl3 is placed in a closed vessel at 80°C and decomposes until equilibrium is reached. (b) If the flask has a volume of 0.250 L, what is the minimum mass of PH3BCl3(𝑠) that must be added to the flask to achieve equilibrium?

Verified Solution
Key Concepts
Equilibrium Constant (Kc)

Le Chatelier's Principle

Stoichiometry and Concentration Calculations
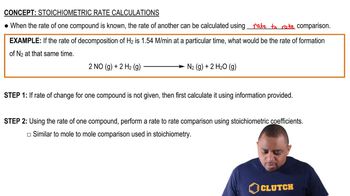
At 218°C, 𝐾𝑐 = 1.2×10−4 for the equilibrium NH4SH(𝑠) ⇌ NH3(𝑔) + H2S(𝑔) Calculate the equilibrium concentrations of NH3 and H2S if a sample of solid NH4SH is placed in a closed vessel at 218°C and decomposes until equilibrium is reached.
At 80°C, 𝐾𝑐 = 1.87×10−3 for the reaction PH3BCl3(𝑠) ⇌ PH3(𝑔) + BCl3(𝑔) (a) Calculate the equilibrium concentrations of PH3 and BCl3 if a solid sample of PH3BCl3 is placed in a closed vessel at 80°C and decomposes until equilibrium is reached.
At 25°C, the reaction CaCrO4(𝑠) ⇌ Ca2+(𝑎𝑞) + CrO42−(𝑎𝑞) has an equilibrium constant 𝐾𝑐 = 7.1×10−4. What are the equilibrium concentrations of Ca2+ and CrO42− in a saturated solution of CaCrO4?
Consider the following equilibrium, for which Δ𝐻<0
2 SO2(𝑔) + O2(𝑔) ⇌ 2 SO3(𝑔)
(f) How will each of the following changes affect an equilibrium mixture of the three gases: SO3(𝑔) is removed from the system?
Consider the reaction 4 NH3(𝑔) + 5 O2(𝑔) ⇌ 4 NO(𝑔) + 6 H2O(𝑔), Δ𝐻 = −904.4 kJ Does each of the following increase, decrease, or leave unchanged the yield of NO at equilibrium? (c) decrease [O2]
