The following mechanism has been proposed for the reaction of NO with H2 to form N2O and H2O: NO1g2 + NO1g2¡N2O21g2 N2O21g2 + H21g2¡N2O1g2 + H2O1g2 (d) The observed rate law is rate = k3NO423H24. If the proposed mechanism is correct, what can we conclude about the relative speeds of the first and second reactions?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Reaction Mechanism
Rate Law

Rate-Determining Step

At 28 C, raw milk sours in 4.0 h but takes 48 h to sour in a refrigerator at 5 C. Estimate the activation energy in kJ>mol for the reaction that leads to the souring of milk.
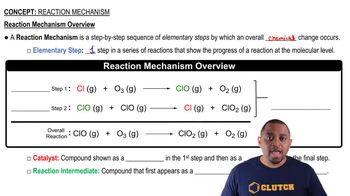
Ozone in the upper atmosphere can be destroyed by the following two-step mechanism: Cl1g2 + O31g2¡ClO1g2 + O21g2 ClO1g2 + O1g2¡Cl1g2 + O21g2 (b) What is the catalyst in the reaction?
The gas-phase decomposition of ozone is thought to occur by the following two-step mechanism.
Step 1: O3(g) ⇌ O2(g) + O(g) (fast)
Step 2: O(g) + O3(g) → 2 O2 (slow)
(b) Derive the rate law that is consistent with this mechanism. (Hint: The product appears in the rate law.)
The gas-phase decomposition of ozone is thought to occur by the following two-step mechanism.
Step 1: O3(g) ⇌ O2(g) + O(g) (fast)
Step 2: O(g) + O3(g) → 2 O2 (slow)
(d) If instead the reaction occurred in a single step, would the rate law change? If so, what would it be?
