The rate of a first-order reaction is followed by spectroscopy, monitoring the absorbance of a colored reactant at 520 nm. The reaction occurs in a 1.00-cm sample cell, and the only colored species in the reaction has an extinction coefficient of 5.60 * 103 M-1 cm-1 at 520 nm. (d) How long does it take for the absorbance to fall to 0.100?
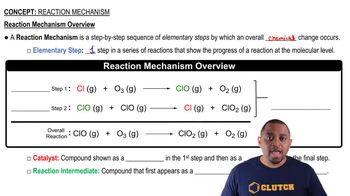
Ozone in the upper atmosphere can be destroyed by the following two-step mechanism: Cl1g2 + O31g2¡ClO1g2 + O21g2 ClO1g2 + O1g2¡Cl1g2 + O21g2 (b) What is the catalyst in the reaction?

Verified Solution
Key Concepts
Catalysts

Ozone Depletion

Reaction Mechanism
At 28 C, raw milk sours in 4.0 h but takes 48 h to sour in a refrigerator at 5 C. Estimate the activation energy in kJ>mol for the reaction that leads to the souring of milk.
The following mechanism has been proposed for the reaction of NO with H2 to form N2O and H2O: NO1g2 + NO1g2¡N2O21g2 N2O21g2 + H21g2¡N2O1g2 + H2O1g2 (d) The observed rate law is rate = k3NO423H24. If the proposed mechanism is correct, what can we conclude about the relative speeds of the first and second reactions?
The gas-phase decomposition of ozone is thought to occur by the following two-step mechanism.
Step 1: O3(g) ⇌ O2(g) + O(g) (fast)
Step 2: O(g) + O3(g) → 2 O2 (slow)
(b) Derive the rate law that is consistent with this mechanism. (Hint: The product appears in the rate law.)
The gas-phase decomposition of ozone is thought to occur by the following two-step mechanism.
Step 1: O3(g) ⇌ O2(g) + O(g) (fast)
Step 2: O(g) + O3(g) → 2 O2 (slow)
(d) If instead the reaction occurred in a single step, would the rate law change? If so, what would it be?
