The density of acetonitrile (CH3CN) is 0.786 g/mL and the density of methanol (CH3OH) is 0.791 g/mL. A solution is made by dissolving 22.5 mL of CH3OH in 98.7 mL of CH3CN. (c) Assuming that the volumes are additive, what is the molarity of CH3OH in the solution?
Calculate the number of moles of solute present in each of the following aqueous solutions: (b) 86.4 g of 0.180 m KCl,

Verified Solution
Key Concepts
Molarity (M)

Moles of Solute
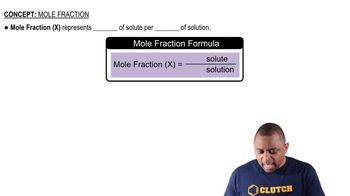
Mass and Molar Mass

The density of toluene (C7H8) is 0.867 g/mL, and the density of thiophene (C4H4S) is 1.065 g/mL. A solution is made by dissolving 8.10 g of thiophene in 250.0 mL of toluene. (a) Calculate the mole fraction of thiophene in the solution.
The density of toluene (C7H8) is 0.867 g/mL, and the density of thiophene (C4H4S) is 1.065 g/mL. A solution is made by dissolving 8.10 g of thiophene in 250.0 mL of toluene. (b) Calculate the molality of thiophene in the solution.
Calculate the number of moles of solute present in each of the following aqueous solutions: (c) 124.0 g of a solution that is 6.45% glucose (C6H12O6) by mass.
Calculate the number of moles of solute present in each of the following solutions: (a) 255 mL of 1.50 M HNO3(aq),
Describe how you would prepare each of the following aqueous solutions, starting with solid KBr: (b) 125 g of 0.180 m KBr,
