Here we're going to talk about a bomb calorimeter. Now, a bomb calorimeter is a steel container with a combustible sample submerged in a known quantity of water. And we're going to say with a bomb calorimeter we have the concept of constant volume calorimetry, and this is used to determine the heat released during a combustion reaction. Now here with this whole idea, we also have constant volume itself. This just means that the calorimeter has a fixed volume that doesn't expand after the sample is combusted.
So just think about combustion reaction as kind of a controlled explosion. So there's an explosion that goes on within the bomb calorimeter itself, but the volume stays fixed, it does not expand outward. And we're going to say, since it's a combustion reaction, that would mean that it's exothermic. This would mean that our enthalpy of standard combustion for this reaction would be equal to -Qlost. So -Q, the amount of heat that's lost by the reaction.
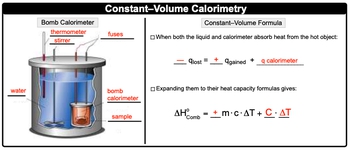
Now if we take a look here, we're going to first look at the bomb calorimeter itself, its components. So with the bomb calorimeter, we're going to have a top, these two wires here. These are actually the fuses that help to ignite the combustion reaction. We're going to say here that they go down here and inside of this orange box, this represents our combustible sample. So we'll just say that this is our sample. This reaction occurs at some designated temperature, and we know that through the use of a thermometer. So here our thermometers saying it's happening at 50�C.
Here we're going to say that this blue outline here is actually our water and the heat that's combusted that's released by our sample. We want to make sure that heat is evenly distributed throughout the water sample, so we use this stirrer and then finally itself. We have our bomb calorimeter. So our bomb calorimeter, remember the sample is combusted, gives off heat. The heat exits the bomb calorimeter, and it goes into the water. The water there, its temperature is read by the thermometer. The heat is dissipated evenly throughout the use of the stirrer.
Now here with the bomb calorimeter, remember we also have our constant volume formula. Here we're going to say this is when both the liquid and calorimeter absorb heat from the hot object. Here we're going to say that it's equal to -Qlost. So the amount of heat loss equals the amount of heat gained. So gaining heat means Q is positive plus the calorimeter itself. And remember we said that standard enthalpy of combustion is equal to -Qlost. So here these are the same. So -Qlost equals our standard enthalpy of combustion.
We're going to say that this amount of heat gained is related to this portion since the heat gain is positive that this MCAT is also positive. And then we're going to finally say that our calorimeter itself, it uses our heat capacity which is capital C times the change in temperature. We would say that this portion when we look at it is the constant volume formula where our standard enthalpy of combustion equals positive MCAT plus our heat capacity times change in temperature.