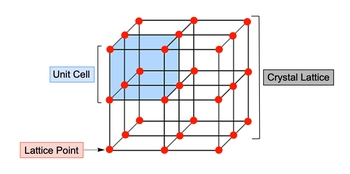
Now, when we use the term crystalline solids, just realize that crystalline solids are closely packed structures arranged in a highly ordered and symmetrical pattern, and with them there are three key terms you need to keep in mind. First, a unit cell. We're going to say a unit cell is the smallest and most fundamental repeating structure of a crystal lattice, and a crystal lattice itself represents identical unit cells arranged in a three-dimensional space.
And with that we have lattice points which are just areas within a crystal lattice which is occupied by an atom with identical surroundings. So here, if we take a look at a crystalline solid, we're going to say that the entire structure here, which looks like 8 cubes stacked with each other, this whole entire structure represents our crystal lattice. Our unit saw is one of those individual cubes.
So here this would be our unit cell and the edges and points of each of those cells. That is our lattice point. So just realize when you're looking at a crystalline solid, the crystalline solid can be seen as the macro view of it. And then we're going towards the lattice point, which is the micro the smallest portion of a unit cell. So just keep these terms in mind when looking at any type of crystalline solid.