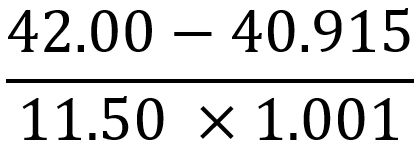So now we're going to see how significant figures can be incorporated in different calculations that will be exposed to in chemistry. Now we're going to start out with multiplication and division. We're going to say when either multiplying or dividing different numbers, the final answer will contain the least significant figures.
And if we take a look at this example, it says perform the following calculation to the right number of sig figs. Here we have three values that are being multiplied together. We have 3.16 ∗ 0.003027 ∗ 5.7 ∗ 10 - 3. We just said that when you're multiplying or dividing its least number of sig figs for your final answer. So we need to determine the number of sig figs for each value.
From our topic on significant figures we know that if we have a decimal point which all are going to do, we move from left to right. Now remember we're going to start counting once we get to our first non 0 number. Here 3 is our first non 0 number and once we start counting we count all the way into the end, so 123. This has three sigfix for the next one skip, skip, skip. Our first non 0 is this 3 1234. This has four sig figs and then finally we have 5.7 ∗ 10 - 3 written in scientific notation.
Remember when it's written in scientific notation? Just focus on the coefficient. We're going to say our first non 0 number is this 5, and once we start counting we count all the way into the end, so 1-2. This has two sig figs. Now, based on our sig figs of 3-4 and two, we have to go with the least number of significant figures. That means our answer at the end can only have two significant figures.
So when we first get our answer, what we see initially is 5.4522324 ∗ 10 - 5. We want two sig figs here. That four that we have though, we look to the right of it and see if we either keep it as four or we round U next to it. We have this long string of numbers and we have a 5 there. Because that number is five. That means we have to round up so the 5.4 becomes now 5.5 and then times 10 - 5.
This represents our answer, which has the least number of significant figures based on the initial values given. We were given these three numbers initially, and the one with the least number of sig figs was the one written in scientific notation. So that tells me that my final answer has to have that number of significant figures. Now that we've looked at multiplication and division, let's go on to our next video and let's see what happens when we incorporate addition and subtraction.