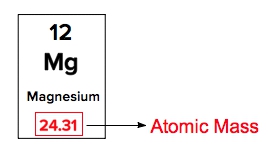
Atomic mass is a crucial concept in chemistry, representing the mass of an element that encompasses the masses of its subatomic particles: protons, neutrons, and electrons. This value can be easily found on the periodic table, where each element is listed with its corresponding atomic mass. For instance, hydrogen, denoted by the symbol H, has an atomic mass of approximately 1.008.
It is important to note that atomic masses are often not whole numbers. This is because the atomic mass of an element is calculated as an average of the masses of its isotopes, which are variants of the element that have the same number of protons but different numbers of neutrons. Consequently, the atomic mass reflects the weighted average of these isotopes based on their natural abundance.
Atomic mass can be expressed in various units, with grams per mole being the most common. Additionally, it can be represented in atomic mass units (amu) or Daltons. To provide context, one atomic mass unit is defined as approximately \(1.66 \times 10^{-27}\) kilograms.
When examining the periodic table, each element is identified by its symbol, with the atomic mass displayed below it. The number above the symbol represents the atomic number, denoted by the variable \(Z\), which indicates the number of protons in the nucleus of an atom. Understanding these components is essential for interpreting the periodic table and grasping the fundamental properties of elements.