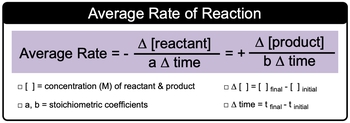
Now average or general rate is the change in concentration in terms of molarity of a reactant or product over a period of time. Now here when we say change in concentrations, realize that the change in concentrations for reactants will be negative in terms of their sign and for products positive. The reason for this is remember as a reaction proceeds reactants are lost or broken down so that we can make product. So products are made overtime with our average or general rate.
We have our average or general rate expression. Here we'd say that rate equals changing concentration over change in time. But if we're applying it to a balanced chemical equation it takes on a little bit more meaning to it. So here we have change in concentration of reactants. Remember change in concentration. Concentration is shown by brackets equals final concentration minus initial concentration divided by A times change in time. Here A represents the stoichiometric coefficients for the reactants.
In a balanced chemical equation this rate would equal the rate of the product formation. Here it equals positive because remember products are being made. Change in product concentration divided by B times change in time. Again, change in product concentration is the same idea, it's final concentration minus initial concentration. Here B would represent again the stoichiometric coefficient, this time in terms of the products and change in time again would be your final time minus your initial time.
We're going to be utilizing these general average rate expressions when looking at a balanced chemical equation and being asked to discuss the relationship in rates between your reactants and your products, right. So keep these ideas in mind as we progress more and more into this idea of changes in concentration of our reactant molecules to produce products.