Consider the three electronic transitions in a hydrogen atom shown here, labeled A, B, and C. (a) Three electromagnetic waves, all drawn on the same scale, are also shown. Each corresponds to one of the transitions. Which electromagnetic wave (i), (ii), or (iii), is associated with electronic transition C?
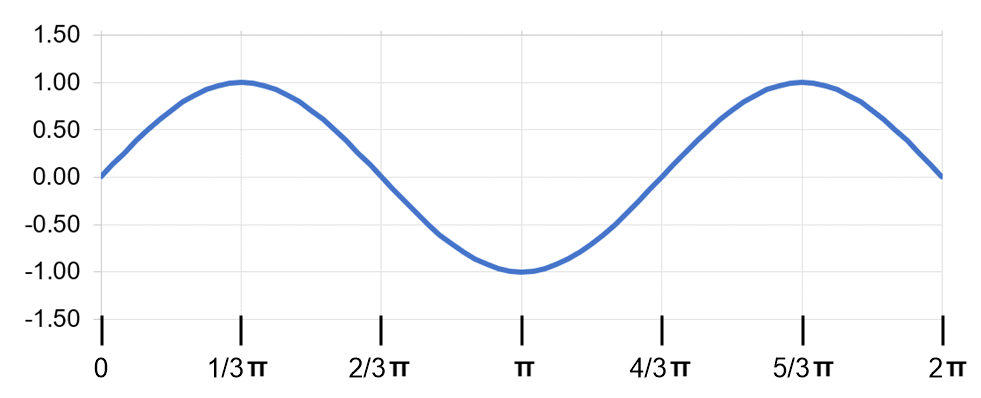
Consider a fictitious one-dimensional system with one electron. The wave function for the electron, drawn below, is c1x2 = sin x from x = 0 to x = 2p. (b) At what value or values of x will there be the greatest probability of finding the electron?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Wave Function

Probability Density

Nodes and Antinodes

Consider the three electronic transitions in a hydrogen atom shown here, labeled A, B, and C. (b) Calculate the energy of the photon emitted for each transition.
Calculate the energy of the photon emitted for transition C.
Consider the three electronic transitions in a hydrogen atom shown here, labeled A, B, and C. (c) Calculate the wavelength of the photon emitted for each transition. Do any of these transitions lead to the emission of visible light? If so which one(s)?
Calculate the wavelength of the photon emitted for transition B.
The contour representation of one of the orbitals for the n = 3 shell of a hydrogen atom is shown here. (a) What is the quantum number l for this orbital?
The contour representation of one of the orbitals for the n = 3 shell of a hydrogen atom is shown here. (c) In which of the following ways would you modify this sketch if the value of the magnetic quantum number, ml, were to change? (i) It would be drawn larger, (ii) the number of lobes would change, (iii) the lobes of the orbital would point in a different direction, (iv) there would be no change in the sketch.
The accompanying drawing shows a contour plot for a dyz orbital. Consider the quantum numbers that could potentially correspond to this orbital. (b) What is the value of the angular momentum quantum number, l?
