Here are the essential concepts you must grasp in order to answer the question correctly.
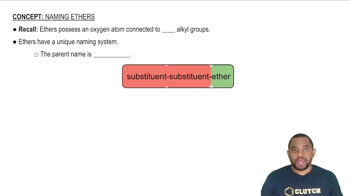
Ethers
Ethers are a class of organic compounds characterized by an oxygen atom bonded to two alkyl or aryl groups. The general formula for ethers is R-O-R', where R and R' represent the hydrocarbon chains. Ethers are known for their relatively low reactivity and are often used as solvents in organic chemistry due to their ability to dissolve a wide range of substances.
Recommended video:
Structural Representation
In organic chemistry, structural representation refers to the way molecules are depicted to show the arrangement of atoms and the bonds between them. Common methods include Lewis structures, condensed formulas, and skeletal formulas. Understanding how to draw these structures is essential for visualizing molecular geometry and predicting chemical behavior.
Recommended video:
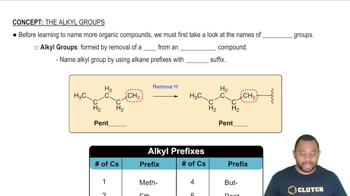
Alkyl Groups
Alkyl groups are derived from alkanes by removing one hydrogen atom, resulting in a group that can be attached to other atoms or groups in a molecule. They are typically represented as 'R' in chemical formulas. In the case of dipentyl ether, the alkyl groups are pentyl groups, which consist of five carbon atoms each, contributing to the overall structure and properties of the ether.
Recommended video:
 Verified step by step guidance
Verified step by step guidance