In Figure 8.10, the energetic coupling of substrate phosphorylation and an endergonic reaction are shown. If the hydrolysis of ATP releases 7.3 kcal of free energy, use the graph in this figure to estimate what you would expect the ∆G values to be for the uncoupled reaction and the two steps in the coupled reaction.
Ch. 8 - Energy and Enzymes: An Introduction to Metabolism

Chapter 8, Problem 10
You have discovered an enzyme that appears to function only when a particular sugar accumulates. Which of the following scenarios would you predict to be responsible for activating this enzyme?
a. The sugar cleaves the enzyme to form the active conformation.
b. The sugar is an allosteric regulatory molecule for the enzyme.
c. The sugar is a competitive inhibitor for the enzyme.
d. The sugar phosphorylates the enzyme to form the active conformation.
 Verified step by step guidance
Verified step by step guidance1
Understand the role of the sugar in enzyme activation. The problem suggests that the enzyme functions only when a particular sugar accumulates, indicating that the sugar plays a role in activating the enzyme.
Consider the concept of allosteric regulation. Allosteric regulation involves a molecule binding to a site other than the active site on an enzyme, causing a conformational change that affects enzyme activity. This is a common mechanism for enzyme activation.
Evaluate the option of the sugar being an allosteric regulatory molecule. If the sugar acts as an allosteric regulator, it would bind to the enzyme at a site other than the active site, inducing a conformational change that activates the enzyme.
Analyze the other options: a) Cleavage of the enzyme by the sugar would imply a permanent change, which is less common for reversible regulation. c) Competitive inhibition involves the sugar competing with the substrate for the active site, which would inhibit rather than activate the enzyme. d) Phosphorylation typically involves the addition of a phosphate group, not a sugar, to activate an enzyme.
Conclude that the most likely scenario is that the sugar acts as an allosteric regulatory molecule, as this aligns with the reversible and regulatory nature of enzyme activation in response to the accumulation of a specific molecule.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Allosteric Regulation
Allosteric regulation involves the binding of a molecule at a site other than the enzyme's active site, causing a conformational change that affects enzyme activity. This can either activate or inhibit the enzyme. In the context of the question, if the sugar acts as an allosteric regulator, it would bind to the enzyme and induce a change that activates the enzyme.
Recommended video:
Guided course

Cell Cycle Regulation
Competitive Inhibition
Competitive inhibition occurs when a molecule similar in structure to the substrate competes for binding at the enzyme's active site. This prevents the actual substrate from binding, thereby inhibiting enzyme activity. In the scenario described, if the sugar were a competitive inhibitor, it would not activate the enzyme but rather block its function.
Recommended video:
Guided course

Enzyme Inhibition
Enzyme Phosphorylation
Phosphorylation is a common post-translational modification where a phosphate group is added to an enzyme, often resulting in a functional change. This can activate or deactivate the enzyme. If the sugar phosphorylates the enzyme, it could potentially activate the enzyme by altering its conformation to a more active state.
Recommended video:
Guided course
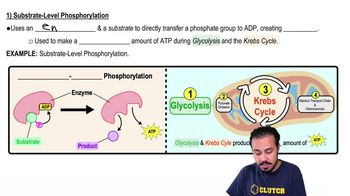
Substrate-Level Phosphorylation
Related Practice
Textbook Question
1685
views
Textbook Question
Using what you have learned about changes in Gibbs free energy, would you predict the ∆G value of catabolic reactions to be positive or negative? What about anabolic reactions? Justify your answers using the terms 'enthalpy' and 'entropy.'
1965
views
Textbook Question
Draw a chemical equation to represent the redox reaction that occurs when methane (CH4) burns in the presence of oxygen (O2). Identify the reactant that is reduced and the reactant that is oxidized. Of the four molecules that should be in your equation, point out the one that has bonds with the highest potential energy.
1929
views
1
rank
