In this video, we're going to introduce some specific disaccharides. Recall that disaccharides are defined as sugars that consist of exactly 2 monosaccharides; 'di' means 2 and 'saccharides' means sugars. There are many disaccharides that exist in nature, so you're definitely not expected to memorize all of them. However, the most abundant disaccharides are the following four: maltose, cellobiose, lactose, and sucrose.
Notice in our image, we're just giving you information about these four disaccharides. The disaccharide consists of a sugar 1 plus a sugar 2, a glycosidic linkage that is linking those two sugars. In this table, we have sugar 1 in the first row, sugar 2 in the second, and the glycosidic linkage in the third. We then put the name of the disaccharide in this column, provide a bit of information about the disaccharide, and let you know if the disaccharide is digestible or not with check boxes. Also, notice that the table is color-coordinated to the structures of the disaccharides shown below; the yellow corresponds with the yellow, green with the green, white with the white, and blue with the blue.
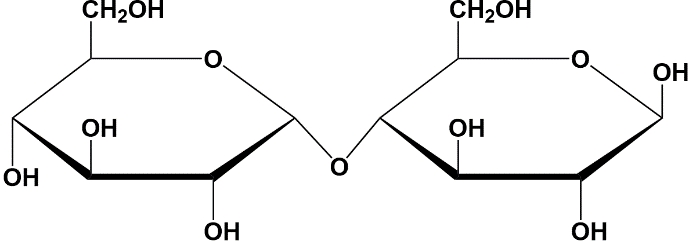
Let's get started with maltose. Notice that maltose has a glycosidic linkage used to link together these two D-glucose residues, as identified here in this image, which is an alpha 1-4 glycosidic linkage. Maltose, found in the polysaccharide starch, is digestible in many plant-based foods including potatoes.
The next disaccharide is cellobiose. It consists of the same sugar residues as maltose, the two D-glucose residues. Instead of having an alpha 1-4 glycosidic linkage like maltose, cellobiose has a beta 1-4 glycosidic linkage. Cellobiose is found in cellulose, a polysaccharide that we will discuss later in our course. Despite their similarity, the configuration of the anomeric carbon in the glycosidic linkage makes cellobiose indigestible by most mammals, highlighting the fact that configurations matter significantly for digestibility.
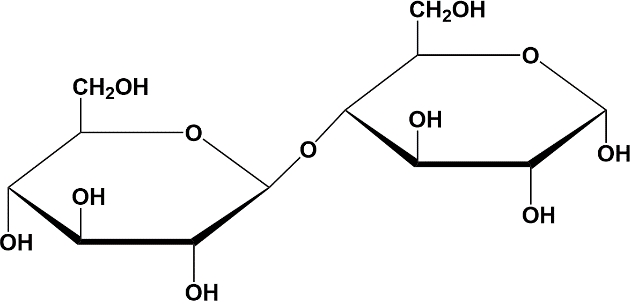
Moving to lactose, this disaccharide swaps the D-glucose residue for a D-galactose residue. However, the second sugar remains a D-glucose, like the others we have covered. The glycosidic linkage in lactose is a beta 1-4 glycosidic linkage. Lactose is commonly found in milk and is digestible by most mammals, but not all due to lactose intolerance, which will be discussed later in our course.
Finally, we discuss sucrose, which retains the D-glucose residue for its first sugar but swaps the second sugar for a D-fructose molecule. The glycosidic linkage in sucrose involves two anomeric carbons, the first being an alpha, and the second, on the same side as its highest numbered carbon, is a beta 1-2 glycosidic linkage. Sucrose, commonly found in processed foods, is digestible by most mammals.
Most of the sugars in our list are D-glucose residues except for D-galactose in lactose and D-fructose in sucrose. In the glycosidic linkages, all except sucrose's are 1-4 linkages. Maltose features an alpha linkage, whereas cellobiose and lactose have beta linkages, and sucrose features both alpha and beta. The only indigestible disaccharide in our list is cellobiose. Spending time with this table and committing these structures and components to memory will help you master these concepts quickly. Moving forward, we'll get some practice applying these concepts. See you in our next video.