Considering that water is a main component of the juices in the stomach and intestines, explain why digestion of lipids is more complicated than digestion of carbohydrates and proteins.
Explain why monosaccharides are polar and fatty acids are nonpolar even though they both contain the same atoms.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Polarity
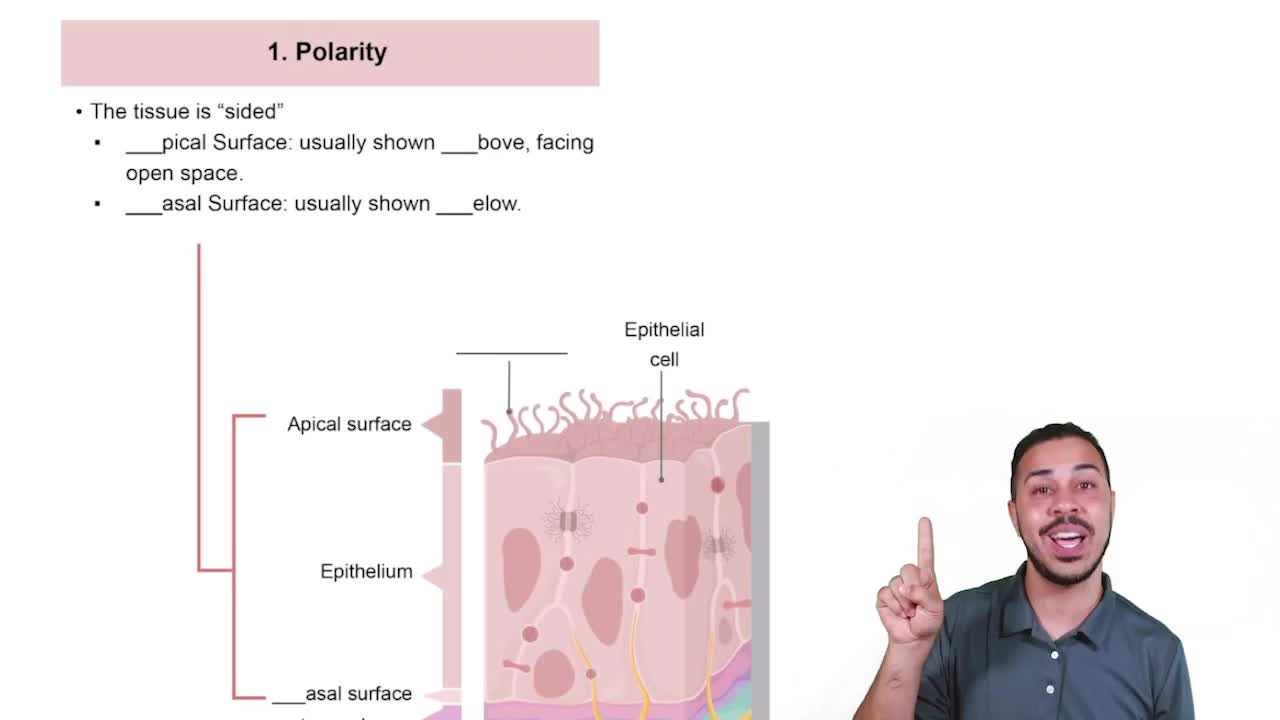
Hydrophilicity vs. Hydrophobicity

Molecular Structure and Functional Groups

Which of the following statements is/are true regarding ATP?
a. It is the body's primary source of chemical energy.
b. Its production is the main reason humans breathe oxygen.
c. It is produced using the energy from the oxidation of molecular fuels like glucose.
d. All of the above statements are true.
Which of the following statements correctly describes a solution?
a. In a solution the solute is chemically dissolved by the solvent.
b. Solutions involve large particles suspended in another component.
c. The particles in a solution will settle out if left to sit.
d. The amount of solute in a solution is expressed as the solution's concentration.
Many drugs and poisons exert their effects by blocking one or more enzymes. How could blocking an enzyme lead to the death of a cell?
Explain the difference between an ionic and a covalent bond
Explain how buffer systems in the body work if the pH of body fluids increases. Is this an example of a negative or a positive feedback loop? Explain. (Connects to Chapter 1)
