Six moles of an ideal gas are in a cylinder fitted at one end with a movable piston. The initial temperature of the gas is °C and the pressure is constant. As part of a machine design project, calculate the final temperature of the gas after it has done J of work.

A gas undergoes two processes. In the first, the volume remains constant at m3 and the pressure increases from Pa to Pa. The second process is a compression to a volume of m3 at a constant pressure of Pa. In a -diagram, show both processes.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Pressure-Volume (pV) Diagram

Isovolumetric Process
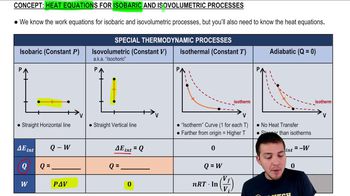
Isobaric Process

The graph in Fig. E shows a -diagram of the air in a human lung when a person is inhaling and then exhaling a deep breath. Such graphs, obtained in clinical practice, are normally somewhat curved, but we have modeled one as a set of straight lines of the same general shape. (Important: The pressure shown is the gauge pressure, not the absolute pressure.) How many joules of net work does this person's lung do during one complete breath?
The graph in Fig. E shows a -diagram of the air in a human lung when a person is inhaling and then exhaling a deep breath. Such graphs, obtained in clinical practice, are normally somewhat curved, but we have modeled one as a set of straight lines of the same general shape. (Important: The pressure shown is the gauge pressure, not the absolute pressure.) The process illustrated here is somewhat different from those we have been studying, because the pressure change is due to changes in the amount of gas in the lung, not to temperature changes. (Think of your own breathing. Your lungs do not expand because they've gotten hot.) If the temperature of the air in the lung remains a reasonable °C, what is the maximum number of moles in this person's lung during a breath?
A gas undergoes two processes. In the first, the volume remains constant at m3 and the pressure increases from Pa to Pa. The second process is a compression to a volume of m3 at a constant pressure of Pa. Find the total work done by the gas during both processes.
In Fig. a, consider the closed loop . This is a cyclic process in which the initial and final states are the same. Find the total work done by the system in this cyclic process, and show that it is equal to the area enclosed by the loop.
Figure E shows a -diagram for an ideal gas in which its absolute temperature at is one-fourth of its absolute temperature at . Did heat enter or leave the gas from to ? How do you know?
