Here are the essential concepts you must grasp in order to answer the question correctly.
Photon Momentum
Photons, despite having no mass, carry momentum, which is given by the formula p = E/c, where p is momentum, E is energy, and c is the speed of light. The momentum of a photon can also be expressed in terms of its wavelength using the relation p = h/λ, where h is Planck's constant and λ is the wavelength. This relationship is crucial for determining the wavelength of a photon when its momentum is known.
Recommended video:
Planck's Constant
Planck's constant (h) is a fundamental constant in quantum mechanics, approximately equal to 6.626 x 10^-34 Js. It relates the energy of a photon to its frequency through the equation E = hν, where ν is the frequency. In the context of photon momentum, it is used to connect momentum and wavelength, making it essential for solving problems involving photons.
Recommended video:
Phase Constant of a Wave Function
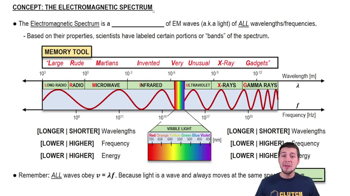
Electromagnetic Spectrum
The electromagnetic spectrum encompasses all types of electromagnetic radiation, ranging from radio waves to gamma rays. Each type of radiation is characterized by its wavelength or frequency. Understanding where a photon lies within this spectrum is important for applications in fields such as telecommunications, medicine, and astronomy, as different regions have distinct properties and uses.
Recommended video:
The Electromagnetic Spectrum