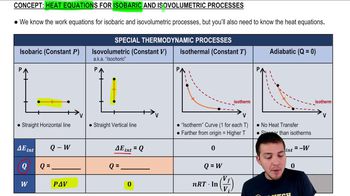
Here are the essential concepts you must grasp in order to answer the question correctly.
Isobaric Process
An isobaric process is a thermodynamic process in which the pressure remains constant while the volume and temperature of the gas may change. In this scenario, the nitrogen gas expands at a constant pressure of 3.0 atm, which means that any changes in volume or temperature do not affect the pressure during the expansion.
Recommended video:
Heat Equations for Isobaric & Isovolumetric Processes
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas through the equation PV = nRT. This law is essential for understanding the behavior of gases under various conditions. In this case, it can help determine how the pressure changes when the volume of the gas triples while maintaining a constant temperature.
Recommended video:
Ideal Gases and the Ideal Gas Law
Charles's Law
Charles's Law states that the volume of a gas is directly proportional to its temperature when pressure is held constant. This principle is relevant here because, as the nitrogen gas expands and its volume triples, the temperature must also change to maintain the pressure constant. Understanding this relationship is crucial for predicting the final state of the gas after expansion.
Recommended video:
 Knight Calc 5th Edition
Knight Calc 5th Edition Ch 19: Work, Heat, and the First Law of Thermodynamics
Ch 19: Work, Heat, and the First Law of Thermodynamics Problem 19
Problem 19 Verified step by step guidance
Verified step by step guidance