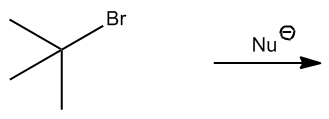All right, guys. Now I want to talk about a really important topic that only applies to the E2 mechanism and that's called the anticoplanar requirement. So as I told you guys already, E2 reactions are going to require an anticoplanar arrangement between the leaving group and the beta hydrogen in order to go to completion. That's because the orbitals need to overlap in a certain way in order to make a new pi bond, which is that double bond that you get at the end. Okay? So that's the first thing that we need to know.
Now, not only are we going to have to look at how many different beta hydrogens we have, but now we're going to have to look at an extra level of complexity which is how many of these beta hydrogens can be in the anti position or are in the anti position. So that means that we're going to require 2 steps to figure out the amount of products that we have. First, we're going to look at beta hydrogens and then after we figure out the number of beta hydrogens, we're going to figure out are they anticoplanar or not.
On top of that, there's one more thing I should know, which is that when you have a leaving group and a beta hydrogen on a cyclohexane, that's actually going to form a chair. Right? Remember that cyclohexanes usually are in the chair conformation. And when you're dealing with an elimination on a chair, instead of calling it anticoplanar, we're actually going to call it a diaxial requirement. Instead of this is the same thing as anticoplanar. Okay? And why is that? Why do I say coplanar? Why do I say diaxial? Because the only way that the leaving group and the beta proton can be anti to each other is if they're on adjacent axial positions.
The reason is because think about the equatorial positions. The axial positions go like this. The equatorial positions go like this. Right? Let me see. I'm doing this all wrong. But let's say that the axial positions are like this. The equatorial positions do this. That's not an anti arrangement. That's actually like a gauche or something like that. Okay? So in order for the elimination to occur, you're going to need to rotate a chair to the axial position first, even though that's the less stable position and that actually has something to do with it as well. Even though this is less stable, I need to rotate it like this in order to make my reaction happen because I need my groups to be anti, not gauche. Okay? So that looks like I was doing a really weird dance, so I hope you guys enjoyed that.
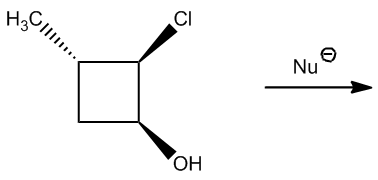
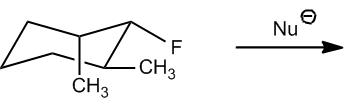
What we're going to do here is a really quick practice, not a lot of drawing. In fact, I don't want you to draw anything yet. All we're analyzing is would these E2 reactions happen or not. Notice that I have a strong nucleophile and I have either a secondary or a tertiary alkyl halide. Remember that I said secondaries and tertiaries can do E2 because they have a bad backside or not that great? So I want you guys to figure out, first of all, like how many beta hydrogens you have. How many beta hydrogens different beta hydrogens would you have. And then once you figure that out, determine would they be anticoplanar or not in order to make the reaction occur. So this is 2 steps. First of all, do the same thing that we did for the beta hydrogen exercise. Figure out how many different ones we have. But then on top of that, figure out how many of those are actually anticoplanar. Okay? And that's going to be the number of possible products for E2. All right. So go ahead and try it with the first one and then I'll explain it.