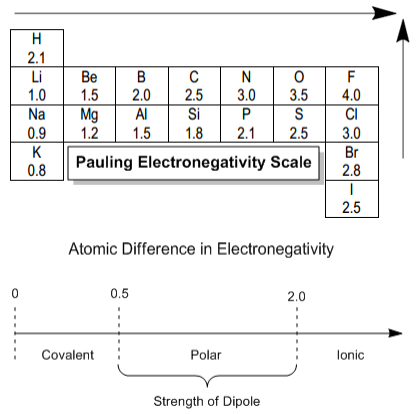
Now I want to talk about one of the most important concepts in all of chemistry, and that's electronegativity. As you guys already know, chemical bonds are formed by the sharing of valence electrons between two atoms. When two atoms share their electrons with each other, that forms a bond. But the extent of that sharing will determine the identity and strength of that bond. What that means is that all bonds are not created equal. Some of them are very, very strong because they have intense sharing, and some of them are very, very weak because they barely have any sharing at all. The unequal sharing of electrons in one direction or another is called a dipole moment. It's calculated based on two variables: the charge and the charge difference between two atoms, and the distance between two atoms. So the charge between any two atoms is going to be related to their difference in electronegativity. Even though we use these two variables to figure out what the dipole moment is, we mainly focus on the charge because the distances are very similar for many bonds. This means the biggest difference is the electronegativity. To figure out what the charge is, we use the Pauling Electronegativity Scale. Although you may see slightly different versions in your book, these differences are minimal.
Fluorine is the most electronegative element, and it all goes downhill from there. Interestingly, hydrogen is unusually electronegative for its position in the periodic table. When discussing bond types, it's not just black and white; there's a spectrum. The nature of the bond is determined by the difference in electronegativities of the two atoms involved. If the difference is less than 0.5, the bond is called covalent, indicating significant electron sharing. In the case of two fluorine atoms, which both have an electronegativity of 4.0, the electrons are equally shared, leading to a pure covalent bond. Then we have polar covalent bonds (partial sharing) for differences between 0.5 to 2.0. As an example, in a carbon and fluorine bond, where carbon has 2.5 and fluorine 4.0 in electronegativity, fluorine will draw more electrons towards itself. The dipole in such situations is represented with an arrow and a line, indicating polar covalent binding.
In polar bonds, partial charges are developed, meaning fluorine will have a partial negative charge and carbon a partial positive, due to unequal electron sharing. Finally, ionic bonds, like NaCl, occur when the difference exceeds typically 2.1. This results in nearly no sharing, as the electrons are localized near the more electronegative atom, represented either by a complete transfer (Na+ and Cl-) or just noted as a bond but recognizing the polarity. The classification of various dipoles includes polar bonds being more ionic or more covalent based on their specific electronegativity differences.
In summary, the study of electronegativity and dipoles is critical in understanding molecular structure and bonding in chemistry. It enables us to predict and rationalize the physical and chemical properties of molecules, which is fundamental in chemical education and research.