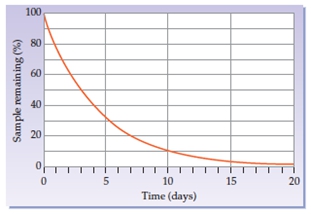
Here we're going to say that half-life, which uses the variable t half, is the amount of time required for half or 50% of a radioisotope to decay. A radioisotope is just the isotopic version of an element that has an unstable nucleus and emits radiation as it decays. Here we're going to say that the radioactive decay of an unknown radioisotope with a half-life of one day is displayed as follows: a half-life is one day, which means that after one day, half of my substance will be gone.
Let's say that we're starting out with some unknown isotope. Remember, our isotope here has x as the symbol, a as the mass number, and z as the atomic number. Let's say that we're starting out with 10 grams of it. Nothing has happened yet, so 0 half-lives have occurred. Initially, I'm starting out with 100% of my substance. Now one day is allowed to pass, and remember, here, a half-life is equal to a day. Now half-lives are not always one day; they vary based on the element. So after one day, I've lost half of my substance, so now I only have 5 grams left. One half-life has passed and all I have left is 50%. I've lost half of what I had.
Another day passes, so I've lost another half. So, starting with 5 grams, another day passes, so now I have only half of that left, which is 2.5 grams. That means 2 half-lives have passed so far, and I only have 25% remaining. Remember, 100% was 10 grams and I only have 2.5 grams. Then another half-life passes, so now I'm losing another half of this 2.5, leaving me with 1.25 grams. So, 3 half-lives have passed, and now I only have 12.5% remaining of the original amount I started with. This is how half-life works, and it keeps going and going and going. I could go to a 4th half-life where I would lose another half of this.
That's the way you have to understand half-life: it's the amount of time it takes to lose half or 50% of my starting material.