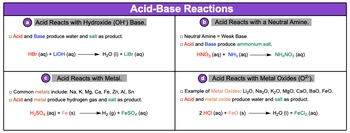
Common acid-base reactions include reactions of acids with:
- a. hydroxide bases, so OH-,
- b. neutral amines,
- c. metals, and
- d. metal oxides.
Now, an amine is just a compound that contains nitrogen and hydrogen or carbon, nitrogen, and hydrogen. And then remember that neutral amines are weak bases. So, if we take a look here at our different types of acid-base reactions, in the first one we have acids reacting with a hydroxide base. We're going to say here, an acid and a base will produce water and a salt as a product. This salt is just an ionic compound.
Here we have an example of an acid-base reaction. Here we'd say that the H+ from the acid combines with the OH- from the base to give us water as a liquid, and then Li+ and Br- will combine together to give me my salt, so LiBr aqueous.
Here we have acids reacting with a neutral amine. So, neutral amines, remember, are weak bases. An example we have NH3 which is ammonia, composed of nitrogen and hydrogen. And here we have what's called methylamine, which has carbon, nitrogen, and hydrogen involved. Here we're going to say that this acid and base together produce a salt. So here we have nitric acid reacting with ammonia. What's going to happen is you're going to create ammonium nitrate as a salt. So remember, this is an ionic compound.
Here we can have acids reacting with metals. So, the common metals can include sodium, potassium, magnesium, calcium, iron, zinc, aluminum, and tin. Here, we're going to say that our acid and metal will produce hydrogen gas, so H2 gas, and they'll also produce a salt.
Alright. So here we have sulfuric acid reacting with a metal; we're going to produce H2 gas, and then the iron will combine with the sulfate to give me iron(II) sulfate aqueous.
Finally, we can have an acid reacting with a metal oxide. Examples of metal oxides include lithium oxide, sodium oxide, potassium, magnesium, calcium, barium, and iron(II) oxide. Here, we're going to say the acid and the metal oxide, they're going to produce water as a product, as well as, again, a salt.
So here, we'd say that we're going to create water from these reacting, and then we'd also create iron(II) chloride. Here we're not really worrying about balancing; we're just looking to see what kind of products we're making from each of these. Remember, these are pattern recognition types of examples. You have to understand what kind of compound is the acid reacting with to help you determine the products that will form.