Here are the essential concepts you must grasp in order to answer the question correctly.
Functional Groups
Benzaldehyde and benzoic acid are both aromatic compounds but differ in their functional groups. Benzaldehyde contains an aldehyde group (-CHO), while benzoic acid has a carboxylic acid group (-COOH). Understanding these functional groups is essential for distinguishing between the two compounds, as they dictate the chemical reactivity and properties of the molecules.
Recommended video:
Functional Group Priorities Concept 1
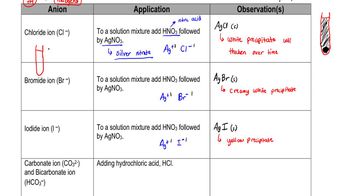
Chemical Tests
Chemical tests are specific reactions used to identify the presence of certain functional groups in organic compounds. For instance, the Tollens' test can be used to detect aldehydes like benzaldehyde, which will reduce silver ions to metallic silver, while benzoic acid does not react. Conversely, benzoic acid can be tested with sodium bicarbonate, which will produce carbon dioxide gas due to the acid's reactivity, whereas benzaldehyde will not.
Recommended video:
Solubility Differences
The solubility of benzaldehyde and benzoic acid in water is another distinguishing factor. Benzoic acid is more soluble in water due to its ability to form hydrogen bonds through its carboxylic acid group, while benzaldehyde is less soluble. This difference can be exploited in separation techniques or tests to differentiate between the two compounds based on their solubility characteristics.
Recommended video:
 Verified step by step guidance
Verified step by step guidance